Chloroform, a colorless liquid that quickly evaporates into a gas, can be pretty harmful. Exposure to this stuff can lead to damage to your eyes, skin, liver, kidneys, and nervous system. And if that wasn’t bad enough, chloroform can also cause cancer if you breathe it in or swallow it. Workers in many industries are at risk of exposure to this toxic substance, including those in the paper manufacturing and recycling industries, service employees who handle some air conditioner refrigerants, and workers at sanitary landfills and water treatment plants.
In recent years, concerns over environmental toxins have been on the rise. Chloroform, a colorless liquid that was once used as an anesthetic during surgery, is one such chemical that has raised alarm bells due to its harmful effects on human health. While the government has set regulations and recommendations to protect public health from exposure to chloroform, the varying not-to-exceed levels among federal organizations are a little complicated. In this article, we’ll explore what chloroform is, how it enters and leaves the body, its potential health effects, and what the government recommends to protect us.
The EPA keeps tabs on the most dangerous waste sites in the country and puts them on the National Priorities List (NPL) for long-term cleanup. Shockingly, chloroform has been found in 717 of the 1,430 current or former NPL sites, including 6 in Puerto Rico and 1 in the Virgin Islands. The scary part is, we don’t even know how many NPL sites have been checked for this toxic substance. So, as more sites are evaluated, we may find even more locations contaminated with chloroform. This matters because exposure to chloroform can be harmful, and these sites may be sources of exposure.
When a substance gets out into the environment from a large industrial plant or a container like a drum or bottle, it doesn’t automatically mean you’re going to be exposed to it. You see, exposure happens only when you come into contact with the substance through breathing it in, eating or drinking it, or touching it with your skin.
If you happen to come into contact with chloroform, there are several factors to take into account that determine whether it’ll do you harm. The amount (or dose) you’re exposed to, the duration of exposure, and the way you’re exposed are just a few examples. But, it’s also important to consider other chemicals you may be exposed to, as well as your age, sex, diet, family traits, lifestyle, and overall health status. All of these factors play a role in determining the potential impact of exposure to chloroform.
What is Chloroform?
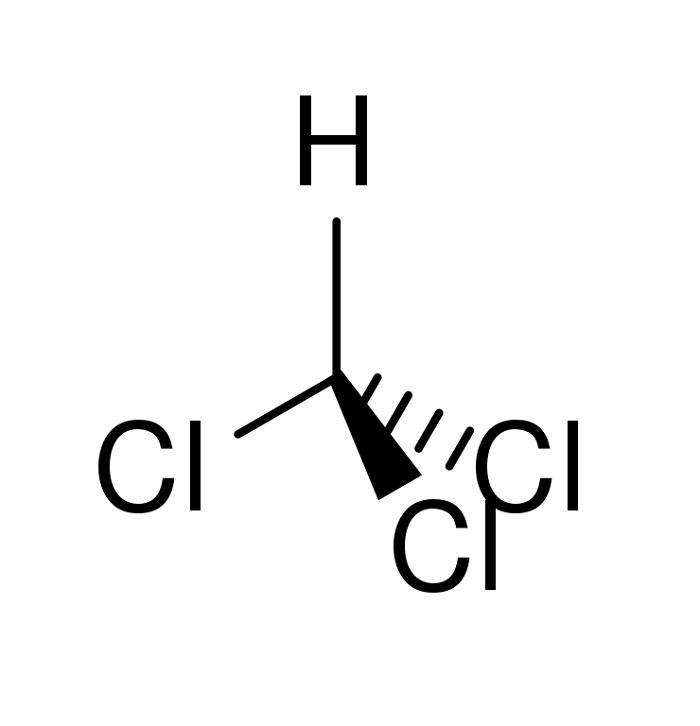
Chloroform, also known as trichloromethane or methyltrichloride, is a colorless liquid with a slightly sweet taste and a pleasant, nonirritating odor. While it can only burn at extremely high temperatures, most of the chloroform found in the environment comes from industrial sources. Although it was once commonly used as an inhaled anesthetic during surgery, it is no longer used for this purpose. Today, most of the chloroform produced in the United States is used to create other chemicals, though some is exported to other countries while we also import chloroform.
Uses of Chloroform
Chloroform is a pretty versatile substance that has been used for various purposes in the past. These include as a solvent for extracting fats, oils, and greases, a dry cleaning spot remover, a fire extinguisher, a fumigant, and even as an anesthetic. However, its use in these products has been discontinued. Nowadays, most of the chloroform produced in the US is used to make HCFC-22 (also known as R-22 or Chlorodifluoromethane), with the rest being exported or used for miscellaneous purposes. HCFC-22 is a colorless gas that is used as a refrigerant in many applications, including residential air conditioning systems1. However, HCFC-22 is being phased out by the U.S. government because it harms the ozone layer.
One of its most significant uses is in the production of monochlorodifluoromethane (CFC-22), a key component of polytetrafluoroethylene (Teflon) and other fluoropolymers. This reaction is carried out with hydrogen fluoride and a small amount of mixed antimony halides, resulting in the formation of chlorodifluoromethane, which is then converted to tetrafluoroethylene, the main precursor to Teflon.
Chloroform is also an excellent solvent due to its ability to participate in hydrogen bonding through the hydrogen attached to carbon. It is used in various applications worldwide, such as a solvent for fats, oils, rubber, waxes, alkaloids, and resins, and as a grain fumigant, cleansing agent, and fire extinguisher. In the rubber industry, it is a vital component in pesticide formulations.
Chloroform is also a Lewis acid that hydrogen bonds with various Lewis bases in solvents such as CCl4 and alkanes. As a reagent, chloroform is a source of the dichlorocarbene :CCl2 group, which is produced by reacting with aqueous sodium hydroxide in the presence of a phase transfer catalyst. This carbene is commonly used in reactions such as the Reimer-Tiemann reaction, where it effects ortho-formylation of activated aromatic rings, and the Kharasch addition, where it forms the CHCl2 free radical in addition to alkenes.
The History of Chloroform
Chloroform has an interesting history, having been independently synthesized by several investigators around 1831. German pharmacist Moldenhawer mixed chlorinated lime with ethanol, while US physician Samuel Guthrie reacted chlorinated lime with ethanol and noted its anesthetic properties. Justus von Liebig carried out the alkaline cleavage of chloral, while Eugène Soubeiran obtained the compound through the action of chlorine bleach on both ethanol and acetone.
In 1834, French chemist Jean-Baptiste Dumas determined chloroform’s empirical formula and named it, and a year later, Dumas prepared the substance by the alkaline cleavage of trichloroacetic acid. Robert Mortimer Glover in London discovered chloroform’s anesthetic qualities on laboratory animals in 1842. Scottish obstetrician James Y. Simpson was the first to demonstrate the anesthetic properties of chloroform on humans in 1847, and helped to popularize the drug for use in medicine. By the 1850s, chloroform was being produced commercially, with about 750,000 doses a week being produced in Britain by 1895. Today, chloroform is prepared on a massive scale through the chlorination of methane and chloromethane, along with dichloromethane.
How Might One Be Exposed to Chloroform?
Chloroform is present in the environment due to the activities of chemical companies, paper mills, and sewage treatment plants. It can also be found in drinking water that has been treated with chlorine, which is added to water and waste water to eliminate bacteria. During the chlorine addition process, small amounts of chloroform are produced as an unintended byproduct. Airborne emissions of chloroform may occur from factories that use or produce it or from its evaporation from soil and water. Additionally, waste water that contains chlorine can release chloroform into water or soil, and spills and leaks from storage and waste sites can also lead to its presence in water and soil. Given the various ways in which chloroform can enter the environment, it is likely present in small quantities almost everywhere.
When Chloroform Enters the Environment, What Happens?
Chloroform behaves differently when it enters the environment. It quickly evaporates in the air, but dissolves easily in water and can travel through soil to reach groundwater. This means that it can contaminate water supplies. Chloroform can remain in the air and groundwater for a long time, but eventually, it breaks down into other substances. However, the process of breaking down is slow, and it can result in more toxic compounds such as phosgene and hydrogen chloride. Chloroform does not accumulate in plants and animals, but small amounts of it may be present in foods.
How Might One Be Exposed to Chloroform?
You could be exposed to small amounts of chloroform in your drinking water, as well as in beverages like soft drinks that use water containing chloroform. Eating food, breathing air, and skin contact with water containing chloroform can also lead to exposure. Drinking water and breathing indoor or outdoor air are the most common ways to be exposed to chloroform. The concentration of chloroform in the air normally ranges from 0.02 to 0.05 ppb, and in treated drinking water, it ranges from 2 to 44 ppb. However, in some areas, the concentration of chloroform may be higher. Chloroform has been found in the air from all over the United States and in almost all public drinking water supplies. It’s estimated that surface water contains 0.1 ppb of chloroform, untreated groundwater contains 0.1 ppb, and soil contains 0.1 ppb. Higher levels of chloroform have been found near hazardous waste sites.
On a typical day, the average amount of chloroform that you might inhale in various places ranges from 2 to 5 micrograms per day (µg/day) in rural areas, 6 to 200 µg/day in cities, and 80 to 2,200 µg/day in areas near major sources of the chemical. The amount of chloroform you are exposed to through drinking water is estimated to range from 4 to 88 µg/day. It’s difficult to estimate the amounts of exposure through eating food and contact with water containing chloroform. Swimmers may absorb chloroform through their skin, while workers in chemical plants and factories that make or use chloroform, as well as those in drinking-water treatment plants, waste water treatment plants, and paper and pulp mills may be exposed to higher than normal amounts. The National Institute for Occupational Safety and Health (NIOSH) estimated that 95,778 workers in the United States have occupational exposure to chloroform.
How Can Chloroform Enter and Leave the Body?
Chloroform can enter your body through various routes, including breathing in air, eating food, drinking water or by skin contact. When you bathe or shower in water containing chloroform, it can easily enter your body through the skin and can also be inhaled as steam. Once inside your body, chloroform is quickly absorbed into your bloodstream from your lungs or intestines. It is then carried by the blood to different parts of your body, such as the liver, fat, and kidneys. Chloroform mostly accumulates in your body fat, but it can also be metabolized into other chemicals, which can cause harmful effects if they accumulate in high amounts inside your body. Some of these metabolites leave your body when you breathe out, while only a small amount leaves the body in the urine and stool.
How Can Chloroform Affect One’s Health?
As we learn more about chloroform and its potential health effects, scientists use a variety of tests to protect public health and find ways to treat those who have been exposed. This may include studying how the chemical is absorbed, used, and released by the body, as well as animal testing to identify potential health effects such as cancer or birth defects. While animal testing may be necessary in some cases, scientists also have a responsibility to treat research animals with care and compassion. Laws exist to protect the welfare of research animals and scientists must comply with strict animal care guidelines. By continuing to study the effects of chloroform and other toxic chemicals, we can make informed decisions to protect public health.
Chloroform can have harmful effects on the central nervous system (brain), liver, and kidneys in humans who breathe air or consume liquids containing high concentrations of the chemical. For many years, chloroform was used as an anesthetic during surgery before its negative impact on the liver and kidneys was discovered. Short-term exposure to approximately 900 parts of chloroform in a million parts of air (900 ppm or 900,000 ppb) can cause fatigue, dizziness, and headaches. If a person is exposed to elevated levels of chloroform in the air, food, or drinking water over an extended period, it can lead to liver and kidney damage. Moreover, exposure to large amounts of chloroform can result in skin sores.
It’s uncertain whether chloroform can lead to reproductive issues or birth defects in humans. However, studies on rats and mice showed that inhaling air with high levels of chloroform (ranging from 30 to 300 ppm) during pregnancy caused miscarriages. Ingesting chloroform during pregnancy also led to miscarriages in rats. In another study, mice that inhaled air with a high level of chloroform (400 ppm) for a few days had abnormal sperm. Additionally, offspring of rats and mice that inhaled chloroform during pregnancy exhibited birth defects.
Studies conducted on people who consumed chlorinated water have suggested a potential connection between the chloroform found in such water and the occurrence of colon and urinary bladder cancer. Meanwhile, rats and mice who ingested food and water with significant levels of chloroform over extended periods developed liver and kidney cancer. Whether or not liver and kidney cancer can also develop in humans after long-term exposure to chloroform in drinking water is unknown. The Department of Health and Human Services has determined, based on animal studies, that chloroform may be expected to cause cancer. The International Agency for Research on Cancer has labeled chloroform as a substance that possibly causes cancer in humans, while the EPA considers chloroform to be a probable human carcinogen.
Are There Any Medical Tests to Determine Exposure to Chloroform?
As of now, there is no reliable test available to measure the amount of chloroform you may have been exposed to or whether it will cause any adverse health effects. Although we can measure the amount of chloroform in the air, blood, urine, and body tissues, these tests are only helpful for a short time after exposure since chloroform leaves the body quickly. Furthermore, since chloroform is a breakdown product of other chemicals, its presence in your body might not necessarily indicate exposure to it specifically, but rather to those chemicals. Therefore, even if you have a small amount of chloroform in your body, it could be due to exposure to those other chemicals and not necessarily from low levels of chloroform in the environment. Blood tests may show if your liver has been damaged, but it cannot determine if chloroform was the cause of the damage.
How Does the Federal Government Protect Human Health As it Relate to Chloroform?
The government plays a crucial role in safeguarding public health through developing regulations and recommendations. Regulations carry the weight of the law and can be enforced by federal agencies such as the Environmental Protection Agency (EPA), the Occupational Safety and Health Administration (OSHA), and the Food and Drug Administration (FDA) in regulating toxic substances. On the other hand, recommendations offer valuable guidance in protecting public health, but they do not have the force of law. Federal organizations such as the Agency for Toxic Substances and Disease Registry (ATSDR) and the National Institute for Occupational Safety and Health (NIOSH) develop these recommendations.
When it comes to regulations and recommendations for toxic substances, they often set what’s called “not-to-exceed” levels in air, water, soil, or food. These levels are usually based on the impact they have on animals, and then they’re adjusted to help protect us humans. Now, here’s the thing: these not-to-exceed levels might vary among different federal organizations. That’s because they might use different exposure times (like an 8-hour workday versus a 24-hour day), rely on different animal studies, or take other factors into account.
Regulations and recommendations are constantly evolving as more information becomes available. To ensure you have the most up-to-date information, be sure to check with the federal agency or organization responsible for providing it. When it comes to chloroform, there are specific regulations and recommendations in place to protect public health.
For instance, the EPA sets limits on the amount of chloroform allowed in water. The EPA limit for total trihalomethanes, which includes chloroform, is 100 micrograms per liter (µg/L, 1 µg/L = 1 ppb in water) in drinking water. Additionally, the EPA requires that spills of 10 pounds or more of chloroform into the environment be reported to the National Response Center.
OSHA is responsible for setting safe levels of chloroform in workplace air across the United States. The maximum permissible occupational exposure limit is 50 parts per million (ppm) or 240 milligrams per cubic meter (mg/m³) in air during an 8-hour workday over a 40-hour workweek. This is considered the ceiling value and is enforced to ensure the health and safety of workers.
The OSHA and EPA limits are regulatory, whereas NIOSH numbers are advisory.
Chloroform is suspected of causing cancer (i.e., possibly carcinogenic, IARC Group 2B) as per the International Agency for Research on Cancer (IARC) Monographs. [PDF]
It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002), and is subject to strict reporting requirements by facilities that produce, store, or use it in significant quantities
References:
- https://www.cdc.gov/niosh/topics/chloroform/default.html
- https://www.atsdr.cdc.gov/
- https://www.atsdr.cdc.gov/ToxProfiles/tp6-c1.pdf
- https://www.epa.gov/sites/default/files/2016-09/documents/chloroform.pdf
- https://en.wikipedia.org/wiki/Chloroform
- chloroform.pdf (epa.gov)
- Residential Air Conditioning and the Phaseout of HCFC-22: What You Need to Know (epa.gov)
- https://www.osha.gov/chemicaldata/477