Respirators and surgical masks are two types of personal protective equipment (PPE) that are crucial for safeguarding workers in healthcare settings. Understanding the distinction between these two forms of PPE is essential for both employees and employers. In this article, we will review the significant differences between respirators and surgical masks, as well as discuss their proper usage and applications.
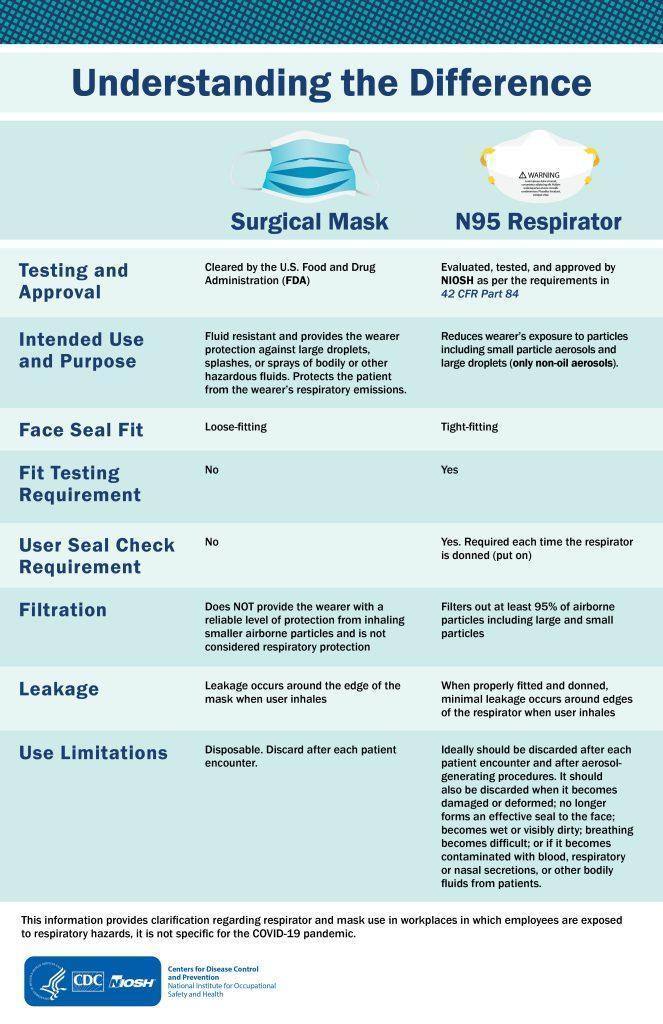
A respirator is a PPE designed to reduce exposure to airborne contaminants. These devices come in various types and sizes, and they must be individually selected to ensure a tight seal with the user’s face. This seal forces inhaled air to pass through the respirator’s filter material, rather than through gaps between the face and the device.
Using a respirator requires compliance with OSHA’s Respiratory Protection standard, 29 CFR 1910.134. This entails a comprehensive respiratory protection program that includes medical evaluation, fit testing, and training elements. Respirators are routinely used to protect healthcare workers against airborne infectious diseases, such as tuberculosis, anthrax, SARS, and Hantavirus, as they guard against both large and small particles.
Facemasks, on the other hand, are loose-fitting, disposable masks that cover the nose and mouth. They include surgical masks, dental masks, medical procedure masks, isolation masks, and laser masks. Facemasks help prevent the spread of large droplets from the person wearing them, whether they are a patient or a healthcare worker. They also protect against splashes or sprays reaching the wearer’s mouth and nose. However, facemasks do not offer protection against the inhalation of small airborne contaminants.
Only facemasks cleared by the U.S. Food and Drug Administration (FDA) may be legally marketed in the United States. FDA approval indicates that the masks have been tested for their ability to resist blood and other body fluid splashes.
Both facemasks and respirators must be worn correctly and consistently to provide adequate protection. When used properly, they play a vital role in preventing exposure to various hazards. If protection from both airborne contaminants and body fluid splashes is necessary, a surgical N95 respirator can be used. These respirators are approved by both NIOSH and the FDA.
During infectious disease outbreaks, such as SARS or pandemic flu, facemasks and respirators should be used in conjunction with other preventive measures. These may include engineering and administrative controls, like installing sneeze guards or allowing teleworking, as well as work practices such as cough etiquette, hand hygiene, and avoiding large gatherings.
It is vital for healthcare workers and their employers to recognize the differences between respirators and surgical masks, as well as understand their proper usage.
References: